Ýëåêòðîííûå êíèãè
Ôîðìàò ïîñìîòðà: Ïîëíûé ìíîãîñòðî÷íûé, Ïîëíûé â äâå ñòðî÷êè, Ïîëíûé áåç àííîòàöèè, Íàèìåíîâàíèå-Öåíà-Àâòîð, Íàèìåíîâàíèå-Öåíà
Ñîðòèðîâêà: Íàèìåíîâàíèå, Öåíà, Àâòîð
 Ðåêëàìà Litres.ru | Combinatorial Reasoning. An Introduction to the Art of Counting Àâòîð: DeTemple Duane Written by two well-known scholars in the field, Combinatorial Reasoning: An Introduction to the Art of Counting presents a clear and comprehensive introduction to the concepts and methodology of beginning combinatorics. Focusing on modern techniques and applications, the book develops a variety of effective approaches to solving counting problems. Balancing abstract ideas with specific topical coverage, the book utilizes real world examples with problems ranging from basic calculations that are designed to develop fundamental concepts to more challenging exercises that allow for a deeper exploration of complex combinatorial situations. Simple cases are treated first before moving on to general and more advanced cases. Additional features of the book include: • Approximately 700 carefully structured problems designed for readers at multiple levels, many with hints and/or short answers • Numerous examples that illustrate problem solving using both combinatorial reasoning and sophisticated algorithmic methods • A novel approach to the study of recurrence sequences, which simplifies many proofs and calculations • Concrete examples and diagrams interspersed throughout to further aid comprehension of abstract concepts • A chapter-by-chapter review to clarify the most crucial concepts covered Combinatorial Reasoning: An Introduction to the Art of Counting is an excellent textbook for upper-undergraduate and beginning graduate-level courses on introductory combinatorics and discrete mathematics. Öåíà: 13587.14 ðóá. ISBN: 9781118833704
Êóïèòü |
 Ðåêëàìà Litres.ru | Communicable Disease Control and Health Protection Handbook Àâòîð: Jeremy Hawker The essential guide to controlling and managing today’s communicable diseases The fourth edition of Communicable Disease Control and Health Protection Handbook offers public health workers of all kinds an authoritative and up-to-date guide to current protocols surrounding the identification and control of infectious diseases. With its concise, accessible design, the book is a practical tool that can be relied upon to explain topics ranging from the basic principles of communicable disease control to recent changes and innovations in health protection practice. Major syndromes and individual infections are insightfully addressed, while the authors also outline the WHO’s international health regulations and the organizational arrangements in place in all EU nations. New to the fourth edition are chapters on Ebola, the Zika virus, and other emerging pandemics. In addition, new writing on healthcare-associated infection, migrant and refugee health, and the importance of preparedness make this an essential and relevant text for all those in the field. This vital resource: Reflects recent developments in the science and administration of health protection practice Covers topics such as major syndromes, control of individual infections, main services and activities, arrangements for all European countries, and much more Includes new chapters on the Zika virus, Schistosomiasis, Coronavirus including MERS + SARS, and Ebola Follows a format designed for ease of use and everyday consultation Created to provide public and environmental health practitioners, physicians, epidemiologists, infection control nurses, microbiologists and trainees with a straightforward – yet informative – resource, Communicable Disease Control and Health Protection Handbook is a practical companion for all those working the field today. Öåíà: 9941.81 ðóá. ISBN: 9781119328056
Êóïèòü |
 Ðåêëàìà Litres.ru | Comparative Politics. Principles of Democracy and Democratization Àâòîð: John Ishiyama T By revealing the contextual conditions which promote or hinder democratic development, Comparative Politics shows how democracy may not be the best institutional arrangement given a country's unique set of historical, economic, social, cultural and international circumstances. Addresses the contextual conditions which promote or hinder democratic development Reveals that democracy may not be the best institutional arrangement given a country's unique set of historical, economic, social, cultural and international circumstances Applies theories and principles relating to the promotion of the development of democracy to the contemporary case studies Öåíà: 3087.48 ðóá. ISBN: 9781444342956
Êóïèòü |
 Ðåêëàìà Litres.ru | Compatibility of Pharmaceutical Solutions and Contact Materials. Safety Assessments of Extractables and Leachables for Pharmaceutical Products Àâòîð: Dennis Jenke Compatibility of Pharmaceutical Products and Contact Materials Dennis Jenke Important safety aspects of compatibility for therapeutic products and their manufacturing systems, delivery devices, and containers Compatibility of Pharmaceutical Products and Contact Materials helps pharmaceutical, toxicology, analytical, and regulatory affairs professionals assess the safety of leachable and extractable chemicals associated with drug product packaging, manufacturing systems, and devices. The most comprehensive resource available, its coverage includes the strategies, tactics, and regulatory requirements for performing safety assessments, along with the means for interpreting results. Structured around a logical framework for an extractables and leachables safety assessment and closely linked to the pharmaceutical product development process, Compatibility of Pharmaceutical Products and Contact Materials directly addresses the fundamental questions of «what activities need to be performed to completely, efficiently, and effectively address the issue of product safety from an extractables and leachables perspective?» and «when do the various required activities need to be performed?» Specifically, the chapters describe: Pertinent regulations and practical ways to meet guidelines Coordinating manufacturing, storage, and delivery systems development and qualification with therapeutic product development Materials characterization and the materials screening process Component and/or system qualification (illustrated by several case studies) Performing validation/migration studies and interpreting and reporting the results Creating a product registration dossier and putting it through regulatory review Product maintenance (Change Control) from an extractables and leachables perspective Likely future developments in extractables and leachables assessment Additionally, the book's appendix provides a database, including CAS registry numbers, chemical formulas and molecular weights of extractable/leachable substances that have been reported in the chemical literature. Detailing the interconnected roles played by analytical chemistry, biological science, toxicology, and regulatory science, Compatibility of Pharmaceutical Products and Contact Materials supplies a much-needed, comprehensive resource to all those in pharmaceutical product or medical device development. Öåíà: 15465.04 ðóá. ISBN: 9780470459409
Êóïèòü |
 Ðåêëàìà Litres.ru | Computational Statistics Àâòîð: Geof H. Givens This new edition continues to serve as a comprehensive guide to modern and classical methods of statistical computing. The book is comprised of four main parts spanning the field: Optimization Integration and Simulation Bootstrapping Density Estimation and Smoothing Within these sections,each chapter includes a comprehensive introduction and step-by-step implementation summaries to accompany the explanations of key methods. The new edition includes updated coverage and existing topics as well as new topics such as adaptive MCMC and bootstrapping for correlated data. The book website now includes comprehensive R code for the entire book. There are extensive exercises, real examples, and helpful insights about how to use the methods in practice. Öåíà: 13991.76 ðóá. ISBN: 9781118555286
Êóïèòü |
 Ðåêëàìà Litres.ru | Computer Applications in Pharmaceutical Research and Development Àâòîð: Sean Ekins A unique, holistic approach covering all functions and phases of pharmaceutical research and development While there are a number of texts dedicated to individual aspects of pharmaceutical research and development, this unique contributed work takes a holistic and integrative approach to the use of computers in all phases of drug discovery, development, and marketing. It explains how applications are used at various stages, including bioinformatics, data mining, predicting human response to drugs, and high-throughput screening. By providing a comprehensive view, the book offers readers a unique framework and systems perspective from which they can devise strategies to thoroughly exploit the use of computers in their organizations during all phases of the discovery and development process. Chapters are organized into the following sections: * Computers in pharmaceutical research and development: a general overview * Understanding diseases: mining complex systems for knowledge * Scientific information handling and enhancing productivity * Computers in drug discovery * Computers in preclinical development * Computers in development decision making, economics, and market analysis * Computers in clinical development * Future applications and future development Each chapter is written by one or more leading experts in the field and carefully edited to ensure a consistent structure and approach throughout the book. Figures are used extensively to illustrate complex concepts and multifaceted processes. References are provided in each chapter to enable readers to continue investigating a particular topic in depth. Finally, tables of software resources are provided in many of the chapters. This is essential reading for IT professionals and scientists in the pharmaceutical industry as well as researchers involved in informatics and ADMET, drug discovery, and technology development. The book's cross-functional, all-phases approach provides a unique opportunity for a holistic analysis and assessment of computer applications in pharmaceutics. Öåíà: 21982.45 ðóá. ISBN: 9780470037225
Êóïèòü |
 Ðåêëàìà Litres.ru | Computer-Aided Pattern Design and Product Development Àâòîð: Bond Terry The use of computers has opened up remarkable opportunities for innovative design, improved productivity, and greater efficiency in the use of materials. Uniquely, this book focuses on the practical use of computers for clothing pattern design and product development. Readers are introduced to the various computer systems which are suitable for the industry, the principles and techniques of pattern design applied to computer systems are explained, and readers are shown how product data management can be used in clothing product development. Öåíà: 7510.67 ðóá. ISBN: 9781405146098
Êóïèòü |
 Ðåêëàìà Litres.ru | Concise Review of Veterinary Microbiology Àâòîð: S. Fanning Updated to reflect the latest developments in the field, Concise Review of Veterinary Microbiology, 2nd Edition, presents essential information on veterinary microbiology for students and those requiring a refresher on key topics relating to microbial diseases in animals. Morphological, cultural and other descriptive features of pathogenic microorganisms are described, together with their habitats and aetiological roles in disease production in animals and, where appropriate, in the human population. Key features: • There are five sections covering bacteriology, mycology, virology, biosecurity and other aspects of infectious diseases • Provides concise, yet comprehensive information on pathogenic microorganisms of importance in veterinary medicine, the diseases which they cause, their diagnosis and control • The 79 short chapters in this book include 13 new chapters on antibacterial resistance, structure and function of the immune system, antifungal chemotherapy, antiviral chemotherapy, principles of biosecurity and a number of topics related to the control and prevention of infectious diseases • This latest edition uses updated nomenclature and includes detailed diagrams now in full colour, and comprehensive tables Öåíà: 4616.57 ðóá. ISBN: 9781118802687
Êóïèòü |
 Ðåêëàìà Litres.ru | Contemporary Accounts in Drug Discovery and Development Àâòîð: Ãðóïïà àâòîðîâ CONTEMPORARY ACCOUNTS IN DRUG DISCOVERY AND DEVELOPMENT A useful guide for medicinal chemists and pharmaceutical scientists Drug discovery is a lengthy and complex process that typically involves identifying an unmet medical need, determining a biological target, chemical library screening to identify a lead, chemical optimization, preclinical studies and clinical trials. This process often takes many years to complete, and relies on practitioners’ knowledge of chemistry and biology, but also—and perhaps more importantly—on experience. Improving the success rate in discovery and development through a thorough knowledge of drug discovery principles and advances in technology is critical for advancement in the field. Contemporary Accounts in Drug Discovery and Development provides drug discovery scientists with the knowledge they need to quickly gain mastery of the drug discovery process. A thorough accounting is given for each drug covered within the book, as the authors provide pharmacology, drug metabolism, biology, drug development, and clinical studies for every case, with modern drug discovery principles and technologies incorporated throughout. Contemporary Accounts in Drug Discovery and Development readers will also find Case histories used as an engaging way of learning about the drug discovery/development process Detailed biological rational and background information, drug design principles, SAR development, ADMET considerations, and clinical studies The full history of individual marketed small molecule drugs Coverage of drug candidates that have passed Phase I clinical trials with different modalities, such as antibody drug conjugates (ADC), proteolysis-targeting chimera (PROTAC), and peptide drugs The application of new technologies in drug discovery such as DNA-encoded libraries (DEL), positron emission tomography (PET), and physics-based computational modeling employing free energy perturbation (FEP) Contemporary Accounts in Drug Discovery and Development is a helpful tool for medicinal chemists, organic chemists, pharmacologists, and other scientists in drug research and process development. It may be considered essential reading for graduate courses in drug discovery, medicinal chemistry, drug synthesis, pharmaceutical science, and pharmacology. It is also a useful resource for pharmaceutical industry labs, as well as for libraries. Öåíà: 19732.89 ðóá. ISBN: 9781119627814
Êóïèòü |
 Ðåêëàìà Litres.ru | Contemporary Accounts in Drug Discovery and Development Àâòîð: Ãðóïïà àâòîðîâ Öåíà: 19732.89 ðóá. ISBN: 9781119627852
Êóïèòü |
 Ðåêëàìà Litres.ru | Contemporary Carbene Chemistry Àâòîð: Robert A. Moss Presents the most innovative results in carbene chemistry, setting the foundation for new discoveries and applications The discovery of stable carbenes has reinvigorated carbene chemistry research, with investigators seeking to develop carbenes into new useful catalysts and ligands. Presenting the most innovative and promising areas of carbene research over the past decade, this book explores newly discovered structural, catalytic, and organometallic aspects of carbene chemistry, with an emphasis on new and emerging synthetic applications. Contemporary Carbene Chemistry features contributions from an international team of pioneering carbene chemistry researchers. Collectively, these authors have highlighted the most interesting and promising areas of investigation in the field. The book is divided into two parts: Part 1, Properties and Reactions of Carbenes, explores new findings on carbene stability, acid-base behavior, and catalysis. Carbenic structure and reactivity are examined in chapters dedicated to stable carbenes, carbodicarbenes, carbenes as guests in supramolecular hosts, tunneling in carbene and oxacarbene reactions, and ultrafast kinetics of carbenes and their excited state precursors. Theoretical concerns are addressed in chapters on computational methods and dynamics applied to carbene reactions. Part 2, Metal Carbenes, is dedicated to the synthetic dimensions of carbenes, particularly the reactions and catalytic properties of metal carbenes. The authors discuss lithium, rhodium, ruthenium, chromium, molybdenum, tungsten, cobalt, and gold. All the chapters conclude with a summary of the current situation, new challenges on the horizon, and promising new research directions. A list of key reviews and suggestions for further reading also accompanies every chapter. Each volume of the Wiley Series on Reactive Intermediates in Chemistry and Biology focuses on a specific reactive intermediate, offering a broad range of perspectives from leading experts that sets the stage for new applications and further discoveries. Öåíà: 17353.58 ðóá. ISBN: 9781118730409
Êóïèòü |
 Ðåêëàìà Litres.ru | Continuous Processing in Pharmaceutical Manufacturing Àâòîð: Ganapathy Subramanian With contributions from biotechnologists and bioengineers, this ready reference describes the state of the art in industrial biopharmaceutical production, with a strong focus on continuous processes. Recent advances in single-use technology as well as application guidelines for all types of biopharmaceutical products, from vaccines to antibodies, and from bacterial to insect to mammalian cells are covered. The efficiency, robustness, and quality control of continuous production processes for biopharmaceuticals are reviewed and compared to traditional batch processes for a range of different production systems. Öåíà: 18726.71 ðóá. ISBN: 9783527673711
Êóïèòü |
 Ðåêëàìà Litres.ru | Convergence Guidebook for Corporate Financial Reporting Àâòîð: Bruce Pounder As a result of the global convergence of financial reporting standards, U.S. GAAP is changing profoundly. U.S. GAAP is also being abandoned by many public and private companies, and will eventually be replaced by a higher-quality set of global standards. The Convergence Guidebook for Corporate Financial Reporting provides the timely, practical guidance that CFOs, controllers, and other financial managers need in order to prepare for the impact of Convergence on their companies, departments, and careers. Guidebook readers will also learn why they must begin preparing for «the next big challenge in corporate financial reporting» now. Öåíà: 7947.93 ðóá. ISBN: 9780470464144
Êóïèòü |
 Ðåêëàìà Litres.ru | Cyclic-Nucleotide Phosphodiesterases in the Central Nervous System. From Biology to Drug Discovery Àâòîð: West Anthony R This book reviews advances in understanding phosphodiesterases within the central nervous system and their therapeutic applications. A range of expert authors from both academia and industry describe these, then focus on the areas of greatest scientific and medical interest to provide more detailed coverage. Therapeutic and drug discovery applications are covered for diseases including Alzheimer's, Parkinson's, schizophrenia, erectile dysfunction, and spinal cord injuries. There is also a chapter on drug discovery tools such as in vitro assays and X-ray structures for medicinal chemistry studies. Öåíà: 14249.93 ðóá. ISBN: 9781118836323
Êóïèòü |
 Ðåêëàìà Litres.ru | Data Mining in Drug Discovery Àâòîð: Ãðóïïà àâòîðîâ Written for drug developers rather than computer scientists, this monograph adopts a systematic approach to mining scientifi c data sources, covering all key steps in rational drug discovery, from compound screening to lead compound selection and personalized medicine. Clearly divided into four sections, the first part discusses the different data sources available, both commercial and non-commercial, while the next section looks at the role and value of data mining in drug discovery. The third part compares the most common applications and strategies for polypharmacology, where data mining can substantially enhance the research effort. The final section of the book is devoted to systems biology approaches for compound testing. Throughout the book, industrial and academic drug discovery strategies are addressed, with contributors coming from both areas, enabling an informed decision on when and which data mining tools to use for one's own drug discovery project. Öåíà: 16726.2 ðóá. ISBN: 9783527656011
Êóïèòü |
 Ðåêëàìà Litres.ru | Data Monitoring Committees in Clinical Trials. A Practical Perspective Àâòîð: Thomas Fleming R The authoritative guide for Data Monitoring Committees—fully revised and updated The number of clinical trials sponsored by government agencies and pharmaceutical companies has grown in recent years, prompting an increased need for interim monitoring of data on safety and efficacy. Data Monitoring Committees (DMCs) are an essential component of many clinical trials, safeguarding trial participants and protecting the credibility and validity of the study. Data Monitoring Committees in Clinical Trials: A Practical Perspective, 2nd Edition offers practical advice for those managing and conducting clinical trials and serving on Data Monitoring Committees, providing a practical overview of the establishment, purpose, and responsibilities of these committees. Examination of topics such as the composition and independence of DMCs, statistical, philosophical and ethical considerations, and determining when a DMC is needed, presents readers with a comprehensive foundational knowledge of clinical trial oversight. Providing recent examples to illustrate DMC principles, this fully-updated guide reflects current developments and practices in clinical trial oversight and offers expanded coverage of emerging issues and challenges in the field. This new second edition covers the most current information on DMC policies, issues in monitoring trials using new designs, and recent trial publications relevant to DMC decision-making. • Presents practical advice for those managing and conducting clinical trials and serving on Data Monitoring Committees • Illustrates the types of challenging issues Data Monitoring Committees face in practical situations • Provides updated and expanded coverage of topics including regulatory and funding agency guidelines and trial designs and their associated demands and limitations • Includes a new chapter addressing legal issues that affect DMC members and discusses general litigation concerns relevant to clinical research • Expands treatment of current journal publications addressing DMC issues Data Monitoring Committees in Clinical Trials: A Practical Perspective, 2nd Edition is a must-have text for anyone engaged in DMC activities as well as trial sponsors, clinical trial researchers, regulatory and bioethics professionals, and those associated with clinical trials in academic, government and industry settings. Öåíà: 15906.9 ðóá. ISBN: 9781119512646
Êóïèòü |
 Ðåêëàìà Litres.ru | Data Science in Theory and Practice Àâòîð: Maria Cristina Mariani DATA SCIENCE IN THEORY AND PRACTICE[/b] EXPLORE THE FOUNDATIONS OF DATA SCIENCE WITH THIS INSIGHTFUL NEW RESOURCE Data Science in Theory and Practice delivers a comprehensive treatment of the mathematical and statistical models useful for analyzing data sets arising in various disciplines, like banking, finance, health care, bioinformatics, security, education, and social services. Written in five parts, the book examines some of the most commonly used and fundamental mathematical and statistical concepts that form the basis of data science. The authors go on to analyze various data transformation techniques useful for extracting information from raw data, long memory behavior, and predictive modeling. The book offers readers a multitude of topics all relevant to the analysis of complex data sets. Along with a robust exploration of the theory underpinning data science, it contains numerous applications to specific and practical problems. The book also provides examples of code algorithms in R and Python and provides pseudo-algorithms to port the code to any other language. Ideal for students and practitioners without a strong background in data science, readers will also learn from topics like: Analyses of foundational theoretical subjects, including the history of data science, matrix algebra and random vectors, and multivariate analysis A comprehensive examination of time series forecasting, including the different components of time series and transformations to achieve stationarity Introductions to both the R and Python programming languages, including basic data types and sample manipulations for both languages An exploration of algorithms, including how to write one and how to perform an asymptotic analysis A comprehensive discussion of several techniques for analyzing and predicting complex data sets Perfect for advanced undergraduate and graduate students in Data Science, Business Analytics, and Statistics programs, Data Science in Theory and Practice will also earn a place in the libraries of practicing data scientists, data and business analysts, and statisticians in the private sector, government, and academia. Öåíà: 13163.15 ðóá. ISBN: 9781119674733
Êóïèòü |
 Ðåêëàìà Litres.ru | Dendrimer-Based Drug Delivery Systems. From Theory to Practice Àâòîð: Tomalia Donald A The opportunities and challenges of using dendrimers to improve drug delivery Among pharmaceutical and biomedical researchers, the use of dendrimers in drug delivery systems has attracted increasing interest. In particular, researchers have noted that the volume of a dendrimer increases when it has a positive charge. If this property can be applied effectively, dendrimers have enormous potential in drug delivery systems, directly supplying medication to targeted human organs. With contributions from an international team of pioneers and experts in dendrimer research, this book provides a comprehensive overview of the latest research efforts in designing and optimizing dendrimer-based drug delivery systems. The book analyzes key issues, demonstrating the critical connections that link fundamental concepts, design, synthesis, analytical methodology, and biological assessment to the practical use of dendrimers in drug delivery applications. Topics covered include: Dendrimer history Synthesis Physicochemical properties Principles of drug delivery Applications in diverse biomedical fields Dendrimer-Based Drug Delivery Systems reflects the authors' thorough review and analysis of the current literature as well as their own firsthand experience in the lab. Readers will not only discover the current state of the science, but also gain valuable insights into fruitful directions for future research. References at the end of each chapter serve as a gateway to the growing body of literature in the field, enabling readers to explore each individual topic in greater depth. Pharmaceutical and biomedical researchers will find this book a unique and essential guide to the opportunities, issues, and challenges involved in fully exploiting the potential of dendrimers to improve drug delivery. Öåíà: 21319.66 ðóá. ISBN: 9781118275207
Êóïèòü |
 Ðåêëàìà Litres.ru | Design, Execution, and Management of Medical Device Clinical Trials Àâòîð: Salah Abdel-aleem M An essential introduction to conducting the various stages of medical device clinical trials Clinical research continues to be one of the most vital components of pharmaceutical, biostatistical, and medical studies. Design, Execution, and Management of Medical Device Clinical Trials provides a uniform methodology for conducting and managing clinical trials. Written in a style that is accessible to readers from diverse educational and professional backgrounds, this book provides an in-depth and broad overview for successfully performing clinical tasks and activities. Throughout the book, practical examples compiled from both the author's and other researchers' previous clinical trial experiences are discussed in a sequential manner as they occur in the study, starting from the development of the clinical protocol and the selection of clinical sites and ending with the completion of the final clinical study report. Next, readers are guided through the development of important clinical documents, including informed consent forms, case report forms, and study logs. A careful review of the Food and Drug Administration (FDA) and International Conference on Harmonisation (ICH) regulations applicable to medical devices is also featured. Additional coverage includes: Qualification and selection of investigators Study monitoring visits Definitions and reporting procedures for adverse events The use of biostatistical methodology in clinical research, including the use of biostatistics for sample size determination and study endpoints The roles and responsibilities of all members of a clinical research team The book concludes with an insightful discussion of special ethical conduct for human research and challenging issues to consider during the design of clinical studies. A glossary lists important clinical and statistical terms used in clinical research, and an extensive reference section provides additional resources for the most up-to-date literature on the topic. Design, Execution, and Management of Medical Device Clinical Trials is an excellent book for clinical research or epidemiology courses at the upper-undergraduate and graduate levels. It is also an indispensable reference for clinical research associates, clinical managers, clinical scientists, biostatisticians, pharmacologists, and any professional working in the field of clinical research who would like to better understand clinical research practices. Öåíà: 12061.29 ðóá. ISBN: 9780470475904
Êóïèòü |
 Ðåêëàìà Litres.ru | Designing and Implementing IP/MPLS-Based Ethernet Layer 2 VPN Services. An Advanced Guide for VPLS and VLL Àâòîð: Zhuo Xu A guide to designing and implementing VPLS services over an IP/MPLS switched service provider backbone Today's communication providers are looking for convenience, simplicity, and flexible bandwidth across wide area networks-but with the quality of service and control that is critical for business networking applications like video, voice and data. Carrier Ethernet VPN services based on VPLS makes this a reality. Virtual Private LAN Service (VPLS) is a pseudowire (PW) based, multipoint-to-multipoint layer 2 Ethernet VPN service provided by services providers By deploying a VPLS service to customers, the operator can focus on providing high throughput, highly available Ethernet bridging services and leave the layer 3 routing decision up to the customer. Virtual Private LAN Services (VPLS) is quickly becoming the number one choice for many enterprises and service providers to deploy data communication networks. Alcatel-Lucent VPLS solution enables service providers to offer enterprise customers the operational cost benefits of Ethernet with the predictable QoS characteristics of MPLS. Items Covered: Building Converged Service Networks with IP/MPLS VPN Technology IP/MPLS VPN Multi-Service Network Overview Using MPLS Label Switched Paths as Service Transport Tunnels Routing Protocol Traffi c Engineering and CSPF RSVP-TE Protocol MPLS Resiliency – Secondary LSP MPLS Resiliency – RSVP-TE LSP Fast Reroute Label Distribution Protocol IP/MPLS VPN Service Routing Architecture Virtual Leased Line Services Virtual Private LAN Service Hierarchical VPLS High Availability in an IP/MPLS VPN Network VLL Service Resiliency VPLS Service Resiliency VPLS BGP Auto-Discovery PBB-VPLS OAM in a VPLS Service Network Öåíà: 7732.52 ðóá. ISBN: 9780470594063
Êóïèòü |
 Ðåêëàìà Litres.ru | Designing the Sustainable Site, Enhanced Edition. Integrated Design Strategies for Small Scale Sites and Residential Landscapes Àâòîð: Venhaus Heather L The full-color, practical guide to designing sustainable residential landscapes and small-scale sites «Going green» is no longer a choice; it's a necessity. Developed landscapes have played a significant role in exacerbating the environmental and social problems that threaten humanity; however, they can also be part of the solution. Designing the Sustainable Site: Integrated Design Strategies for Small-Scale Sites and Residential Landscapes gives site designers and landscape architects the tools and information they need to become a driving force in the quest for sustainability. Advocating a regenerative design approach in which built landscapes sustain and restore vital ecological functions, this book guides readers through a design process for new and redeveloped sites that not only minimizes damage to the environment but also actively helps to repair it. Designing the Sustainable Site: Assists designers in identifying and incorporating sustainable practices that have the greatest positive impact on both the project and the surrounding community, within a regional context Uses photographs, sketches, and case studies to provide a comprehensive look at successful green landscape design Illustrates how sustainable practices are relevant and applicable to projects of any size or budget Demonstrates how built environments can protect and restore ecosystem services Explains the multiple and far-reaching benefits that sustainable design solutions can provide Assists project teams in fulfilling credit requirements of green building assessment tools, such as LEED, BREEAM, or SITES With attention to six global environmental challenges—including air pollution, urban flooding and water pollution, water shortages, invasive species, and loss of biodiversity—along with guidance on how to meet these challenges, Designing the Sustainable Site is a practical design manual for sustainable alternatives to small-scale site and residential landscape design. Öåíà: 7180.2 ðóá. ISBN: 9781118183410
Êóïèòü |
 Ðåêëàìà Litres.ru | Detection and Quantification of Antibodies to Biopharmaceuticals. Practical and Applied Considerations Àâòîð: Michael Tovey G The definitive book on the neutralization of recombinant biopharmaceuticals Recombinant biopharmaceuticals are an important tool for treating a range of illnesses; however, their efficacy can be severely impaired by their immunogenicity. When introduced into the body, these pharmaceuticals can cause the immune system to produce anti-drug antibodies (ADAs) that neutralize their effects. The first and only book to cover neutralization in connection with biopharmaceuticals and the measurement and application of neutralizing antibodies in modern medicine at any real length, Detection and Quantification of Antibodies to Biopharmaceuticals: Practical and Applied Considerations offers a comprehensive and in-depth look at all the principal aspects of the detection and quantification of antibodies that are essential to understanding and responding to the challenges they present. Bringing together a large-scale review of neutralization and biopharmaceuticals and the ability to measure, detect, and apply antibodies to modern science and medicine with international regulatory perspectives, the expectations of regulatory authorities, and the strengths and weaknesses of various assays, the book describes several novel ideas for detecting ADAs. Designed to serve as a resource for biopharmaceutical drug development, the book provides biotechnology companies and pharmaceutical drug development specialists, as well as non-experts, with key insights into the design, optimization, and qualification of assays, the establishment of sampling strategies, the choice of appropriate assay end-points, and data analysis for the detection and quantification of neutralizing antibodies. Öåíà: 17232.47 ðóá. ISBN: 9781118075661
Êóïèòü |
 Ðåêëàìà Litres.ru | Development and Approval of Combination Products Àâòîð: Ãðóïïà àâòîðîâ A step-by-step, integrated approach for successful, FDA-approved combination drug products Using a proven integrated approach to combination drug development, this book guides you step by step through all the preclinical, clinical, and manufacturing stages. Written from an FDA regulatory perspective, the book not only enables you to bring a successful combination drug product to market, it also sets forth the most efficient and effective path to FDA approval. The book begins with an introductory chapter presenting definitions and basic regulatory principles of combination products. Next, it reviews manufacturing and controls, preclinical testing models, pharmacology, clinical testing, regulatory submissions, FDA reviews, and approvals. Among the key topics examined are: * The pharmacology, safety pharmacology, and toxicology supporting human clinical trials of combination products * Approaches to clinical trial protocol design and execution * Chemical, physicochemical, and analytical aspects of manufacturing controls and validation that lead to stable components for combination products * Key sponsor/FDA meetings and negotiations essential for approval and commercialization Case studies involving such actual combination products as Mylotarg, Herceptin, and HercepTest help you better understand how to implement the author's practical guidelines. References at the end of each chapter enable you to find more information on any stage of the development, manufacturing and approval processes. This book is ideal for researchers, regulators, academics, project managers, and executives involved in the complex process of combination product development. Not only does itoffer a comprehensive guide to the technical aspects of the field, it also integrates all ofthese technical aspects into a unified, effective approach to help ensure a successful, approved product. Öåíà: 12145.58 ðóá. ISBN: 9780470371190
Êóïèòü |
 Ðåêëàìà Litres.ru | Development of Therapeutic Agents Handbook Àâòîð: Shayne Cox Gad A comprehensive look at current drug discovery and development methods—and the roadmap for the future Providing both understanding and guidance in characterizing potential drugs and their production and synthesis, Development of Therapeutic Agents Handbook gives professionals a basic tool to facilitate research and development within this challenging process. This comprehensive text brings together, in one resource, a compendium of concepts, approaches, methodologies, and limitations that need to be considered in the formulation of therapeutic agents across a range of therapeutic fields. Both a reference and a call to action for the pharmaceutical industry, Development of Therapeutic Agents Handbook examines recent innovations taking shape in the various medical disciplines involved in drug discovery, and shows why these advances need to be embraced universally among researchers to improve their solution strategies. Additional subject matter includes: Extensive coverage and in-depth look into novel treatments and therapeutics Discussion of hot topics like new drugs and nutraceuticals, the discovery and development of vaccines, cancer therapeutics, and market overviews Coverage of therapeutic drug development for specific disease areas, such as cardiology, oncology, breast cancer, and kidney diseases As research in biology, chemistry, medicine, and technology rapidly progresses, it is becoming increasingly important for medical researchers to maintain an up-to-date knowledge base of emerging trends directing promising new therapies. Development of Therapeutic Agents Handbook serves this purpose, acting as both a one-stop reference rich in valid science, and a tool to carve out new pathways in the pursuit of bringing safer and more effective drugs to the marketplace. Öåíà: 30930.08 ðóá. ISBN: 9781118077108
Êóïèòü |
 Ðåêëàìà Litres.ru | Development of Vaccines. From Discovery to Clinical Testing Àâòîð: Srivastava Indresh K Development of Vaccines: From Discovery to Clinical Testing outlines the critical steps, and analytical tools and techniques, needed to take a vaccine from discovery through a successful clinical trial. Contributions from leading experts in the critical areas of vaccine expression, purification, formulation, pre-clinical testing and regulatory submissions make this book an authoritative collection of issues, challenges and solutions for progressing a biologic drug formulation from its early stage of discovery into its final clinical testing. A section with details and real-life experiences of toxicology testing and regulatory filing for vaccines is also included. Öåíà: 17232.47 ðóá. ISBN: 9781118023624
Êóïèòü |
 Ðåêëàìà Litres.ru | Drug Bioavailability Àâòîð: Ãðóïïà àâòîðîâ The gold standard for industrial research now completely revised in line with current trends in the field, with all contributions extensively updated or rewritten. In 21 chapters readers can benefit from the key working knowledge of today's leading pharmaceutical companies, including Pfizer, AstraZeneca, and Roche. Drug developers from industry and academia present all the factors governing drug bioavailability, complete with practical examples and real-life data. Part I focuses on in vitro and in vivo measurements of physicochemical properties, such as membrane permeability and ionization. Part II discusses solubility and gastrointestinal absorption, while the third part is devoted to metabolism and excretory mechanisms. The much revised and expanded part IV surveys current in silico approaches to predict drug properties needed to estimate the bioavailability of any new drug candidate. The final part shows how poor bioavailability may be improved by various approaches during the development process. No other publication offers the same level of treatment on this crucial topic in modern drug development. Öåíà: 25414.82 ðóá. ISBN: 9783527623877
Êóïèòü |
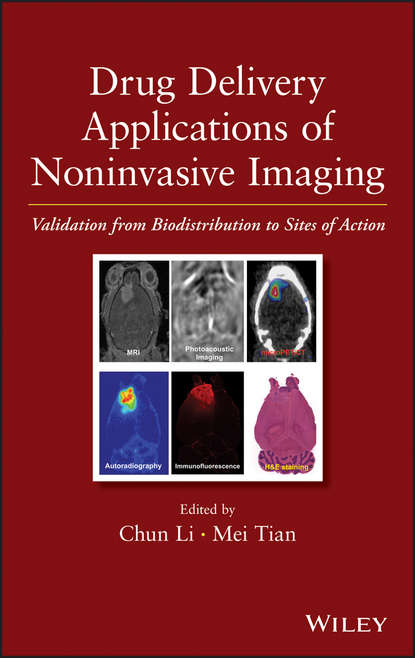 Ðåêëàìà Litres.ru | Drug Delivery Applications of Noninvasive Imaging. Validation from Biodistribution to Sites of Action Àâòîð: Li Chun Cost-effective strategies for designing novel drug delivery systems that target a broad range of disease conditions In vivo imaging has become an important tool for the development of new drug delivery systems, shedding new light on the pharmacokinetics, biodistribution, bioavailability, local concentration, and clearance of drug substances for the treatment of human disease, most notably cancer. Written by a team of international experts, this book examines the use of quantitative imaging techniques in designing and evaluating novel drug delivery systems and applications. Drug Delivery Applications of Noninvasive Imaging offers a full arsenal of tested and proven methods, practices and guidance, enabling readers to overcome the many challenges in creating successful new drug delivery systems. The book begins with an introduction to molecular imaging. Next, it covers: In vivo imaging techniques and quantitative analysis Imaging drugs and drug carriers at the site of action, including low-molecular weight radiopharmaceuticals, peptides and proteins, siRNA, cells, and nanoparticles Applications of imaging techniques in administration routes other than intravenous injection, such as pulmonary and oral delivery Translational research leading to clinical applications Imaging drug delivery in large animal models Clinical applications of imaging techniques to guide drug development and drug delivery Chapters are based on a thorough review of the current literature as well as the authors' firsthand experience working with imaging techniques for the development of novel drug delivery systems. Presenting state-of-the-technology applications of imaging in preclinical and clinical evaluation of drug delivery systems, Drug Delivery Applications of Noninvasive Imaging offers cost-effective strategies to pharmaceutical researchers and students for developing drug delivery systems that accurately target a broad range of disease conditions. Öåíà: 19441.75 ðóá. ISBN: 9781118356821
Êóïèòü |
 Ðåêëàìà Litres.ru | Drug Delivery Approaches Àâòîð: Ãðóïïà àâòîðîâ Öåíà: 22763.25 ðóá. ISBN: 9781119772743
Êóïèòü |
 Ðåêëàìà Litres.ru | Drug Design of Zinc-Enzyme Inhibitors Àâòîð: Ãðóïïà àâòîðîâ Brings together functional and structural informationrelevant to the design of drugs targeting zinc enzymes The second most abundant transition element in living organisms, zinc spans all areas of metabolism, with zinc-containing proteins offering both established and potential drug targets. Drug Design of Zinc-Enzyme Inhibitors brings together functional and structural information relevant to these zinc-containing targets. With up-to-date overviews of the latest developments field, this unique and comprehensive text enables readers to understand zinc enzymes and evaluate them in a drug design context. With contributions from the leaders of today's research, Drug Design of Zinc-Enzyme Inhibitors covers such key topics as: Major drug targets like carbonic anhydrases, matrix metalloproteinases, bacterial proteases, angiotensin-converting enzyme, histone deacetylase, and APOBEC3G Roles of recently discovered zinc-containing isozymes in cancer, obesity, epilepsy, pain management, malaria, and other conditions Cross reactivity of zinc-enzyme inhibitors and activators The extensive use of X-ray crystallography and QSAR studies for understanding zinc-containing proteins Clinical applications An essential resource for the discovery and development of new drug molecules, Drug Design of Zinc-Enzyme Inhibitors gives researchers, professionals, students, and academics the foundation to understand and work with zinc enzyme inhibitors and activators. Öåíà: 26006.69 ðóá. ISBN: 9780470508152
Êóïèòü |
 Ðåêëàìà Litres.ru | Drug Discovery for the Treatment of Addiction Àâòîð: Brian S. Fulton With addiction a key target for drug discovery efforts, this book fills an important and timely need for medicinal chemists who need to understand complex neuroscience issues. The author illustrates medicinal chemistry's prominent role in treating addiction and covers specific drugs of abuse including narcotics, stimulants, depressants, nicotine, and marijuana. • Interprets complex neuro- biological and pharmacological information, like the drug-reward system, for medicinal chemists • Emphasizes neurotransmitters and neurochemical mechanisms of addictive drugs • Pulls together information on the many potential drug targets for treating addiction • Stresses unique medicinal chemistry problems when describing pharmacology testing methods and drug development Öåíà: 12523.93 ðóá. ISBN: 9781118889596
Êóïèòü |
 Ðåêëàìà Litres.ru | Drug Discovery Toxicology Àâòîð: Ãðóïïà àâòîðîâ As a guide for pharmaceutical professionals to the issues and practices of drug discovery toxicology, this book integrates and reviews the strategy and application of tools and methods at each step of the drug discovery process. • Guides researchers as to what drug safety experiments are both practical and useful • Covers a variety of key topics – safety lead optimization, in vitro-in vivo translation, organ toxicology, ADME, animal models, biomarkers, and –omics tools • Describes what experiments are possible and useful and offers a view into the future, indicating key areas to watch for new predictive methods • Features contributions from firsthand industry experience, giving readers insight into the strategy and execution of predictive toxicology practices Öåíà: 20514.15 ðóá. ISBN: 9781119053323
Êóïèòü |
 Ðåêëàìà Litres.ru | Drug Discrimination. Applications to Medicinal Chemistry and Drug Studies Àâòîð: Young Richard Drug discrimination: a practical guide to its contributions to the invention of new chemical entities and evaluations of new or known pharmacological agents Drug discrimination can be described as a «drug detection» procedure that uses a pharmacologically active agent as the subjective stimulus. Although the procedure does require some effort to implement, it can be an extremely important tool for understanding drug action. Whereas medicinal chemists should come to learn the types of information that drug discrimination studies can offer, pharmacologists and psychologists might come to realize how medicinal chemists can apply the types of information that the paradigm routinely provides. Drug Discrimination: Applications to Medicinal Chemistry and Drug Studies provides in-depth analyses of the nature and use of drugs as discriminative stimuli and bridges some of the numerous gaps between medicinal chemistry, pharmacology, and psychology. Stressing the practical aspects of drug discrimination, including types of procedures, study design, data, and interpretation, the book details the advantages and limitations of drug discrimination studies versus other pharmacologic evaluations. Practical information from leading researchers in the field addresses specific topics and techniques that are of interest in drug discovery, evaluation, and development. A groundbreaking new guide to the applications of drug discrimination studies for medicinal chemistry and neuroscience, Drug Discrimination is essential for any scientist, researcher, or student whose interests involve the design, development, and/or action of drugs acting at the level of the central nervous system. Öåíà: 18883.91 ðóá. ISBN: 9781118023136
Êóïèòü |
 Ðåêëàìà Litres.ru | Drug Efficacy, Safety, and Biologics Discovery. Emerging Technologies and Tools Àâòîð: Ekins Sean Drug Efficacy, Safety, and Biologics Discovery: Emerging Technologies and Tools covers key emerging technologies in pharmaceutical R & D and how they have substantially impacted (or are currently impacting) drug discovery. The cross-disciplinary collaborations implicit in integrating these technologies with drug discovery operations will fuel the engine for future innovations. This book cuts across the multiple areas of drug discovery, each chapter authored by pioneers in that field, making for a broad appeal to the chemical and biological scientists and technologists involved in drug discovery and development. Öåíà: 16459.22 ðóá. ISBN: 9780470431801
Êóïèòü |
 Ðåêëàìà Litres.ru | Drug Metabolism Handbook. Concepts and Applications Àâòîð: Ala Nassar F A valuable reference tool for professionals involved in the industry, Drug Metabolism in Pharmaceuticals covers new tools such as LC-MS and LC-MS-NMR along with experimental aspects of drug metabolism. This work fills a gap in the literature by covering the concepts and applications of pharmaceutical research, development, and assessment from the point of view of drug metabolism. By providing both a solid conceptual understanding of the drug metabolism system, and a well illustrated, detailed demonstration and explanation of cutting edge tools and techniques, this book serves as a valuable reference tool for bench scientists, medical students, and students of general health sciences. Öåíà: 24738.54 ðóá. ISBN: 9780470439258
Êóïèòü |
 Ðåêëàìà Litres.ru | Drug Transporters Àâòîð: Ãðóïïà àâòîðîâ DRUG TRANSPORTERS [b]Drug transporter fundamentals and relevant principles and techniques, featuring new and expanded chapters Drug Transporters: Molecular Characterization and Role in Drug Disposition provides in-depth analysis of the conceptual evolution and technical development for studying drug transporters. Contributions by an international panel of leading researchers address advances in transporters as drug targets, transporters in pharmacotherapy, the impact of transporters on drug efficacy and safety, the development of sophisticated model systems and sensitive assay methods, and more. Divided into two parts, the book first provides a thorough overview of relevant drug transporters before detailing the principles of drug transport and associated techniques. The updated and expanded third edition includes new chapters on in vitro-in vivo scale-up of drug transport activities, the ontogeny of drug transporters, the application of physiologically-based pharmacokinetic and pharmacodynamic modeling, and the use of transporters as therapeutic targets for diseases. Reflects the current state of the field and offers perspectives on future directions Covers basic knowledge, clinical outcomes, and emerging discoveries in transporter science Provides up-to-date information on drug transporter families, mechanisms, and clinical implications Includes extensive references and numerous figures and tables throughout Understandable for novices while offering sufficient depth for more experienced researchers, Drug Transporters: Molecular Characterization and Role in Drug Disposition, Third Edition is an excellent textbook for pharmacological or physiological science courses in drug/membrane transport, and an invaluable reference for academic or industrial scientists working in the transporter field and related areas of drug metabolism, pharmacokinetics, and pharmacodynamics. Öåíà: 25296.45 ðóá. ISBN: 9781119739876
Êóïèòü |
 Ðåêëàìà Litres.ru | Drug Utilization Research Àâòîð: Bj?rn Wettermark Drug Utilization Research (DUR) is an eclectic scientific discipline, integrating descriptive and analytical methods for the quantification, understanding and evaluation of the processes of prescribing, dispensing and consumption of medicines and for the testing of interventions to enhance the quality of these processes. The discipline is closely related and linked mainly to the broader field of pharmacoepidemiology, but also to health outcomes research, pharmacovigilance and health economics. Drug Utilization Research is a unique, practical guide to the assessment and evaluation of prescribing practices and to interventions to improve the use of medicines in populations. Edited by an international expert team from the International Society for Pharmacoepidemiology (ISPE), DUR is the only title to cover both the methodology and applications of drug utilization research and covers areas such as health policy, specific populations, therapeutics and adherence. Öåíà: 15382.65 ðóá. ISBN: 9781118949764
Êóïèòü |
 Ðåêëàìà Litres.ru | Drugs of Abuse. Pharmacology and Molecular Mechanisms Àâòîð: Sherrel Howard Drug abuse has been, and continues to be, a global societal issue with diverse sets of impacts. Drugs of Abuse: Pharmacology and Molecular Mechanisms introduces the basic principles of pharmacology and neuroscience of drug abuse. Understanding the chemistry of commonly abused drugs and their impact on brain function will provide students and researchers with a more profound understanding of the molecular basis of drug abuse and addiction. Drugs of Abuse: Pharmacology and Molecular Mechanisms opens with a brief history of drug use and abuse. Subsequent sections look at specific families of drugs, including stimulants, depressants, and hallucinogens among others, and explore how their chemical make-up interacts with brain function. The final chapter provides a brief overview of clinical substance abuse treatment. Providing a concise, accessible introductory overview of the topic, Drugs of Abuse: Pharmacology and Molecular Mechanisms will be a valuable resource for students, researchers, and others interested in how drugs interact with the brain. Introduces readers to the basic principles of neuroscience and pharmacology as related to drug use and abuse. Explores how the chemical make-up of drugs interact with the brain and can lead to addiction Includes coverage of a wide array of commonly abused families of drugs, including stimulants, depressants, hallucinogens, and others. Provides an essential introduction to the chemical and molecular underpinnings of drug use and abuse Öåíà: 9163.04 ðóá. ISBN: 9781118850152
Êóïèòü |
 Ðåêëàìà Litres.ru | Early Drug Development. Strategies and Routes to First-in-Human Trials Àâòîð: Mitchell Cayen N The focus of early drug development has been the submission of an Investigational New Drug application to regulatory agencies. Early Drug Development: Strategies and Routes to First-in-Human Trials guides drug development organizations in preparing and submitting an Investigational New Drug (IND) application. By explaining the nuts and bolts of preclinical development activities and their interplay in effectively identifying successful clinical candidates, the book helps pharmaceutical scientists determine what types of discovery and preclinical research studies are needed in order to support a submission to regulatory agencies. Öåíà: 20104.55 ðóá. ISBN: 9780470613177
Êóïèòü |
 Ðåêëàìà Litres.ru | Electronics in Advanced Research Industries Àâòîð: Alessandro Massaro A one-of-a-kind examination of the latest developments in machine control In Electronics in Advanced Research Industries: Industry 4.0 to Industry 5.0 Advances , accomplished electronics researcher and engineer Alessandro Massaro delivers a comprehensive exploration of the latest ways in which people have achieved machine control, including automated vision technologies, advanced electronic and micro-nano sensors, advanced robotics, and more. The book is composed of nine chapters, each containing examples and diagrams designed to assist the reader in applying the concepts discussed within to common issues and problems in the real-world. Combining electronics and mechatronics to show how they can each be implemented in production line systems, the book presents insightful new ways to use artificial intelligence in production line machines. The author explains how facilities can upgrade their systems to an Industry 5.0 environment. Electronics in Advanced Research Industries: Industry 4.0 to Industry 5.0 Advances also provides: A thorough introduction to the state-of-the-art in a variety of technological areas, including flexible technologies, scientific approaches, and intelligent automatic systems Comprehensive explorations of information technology infrastructures that support Industry 5.0 facilities, including production process simulation Practical discussions of human-machine interfaces, including mechatronic machine interface architectures integrating sensor systems and machine-to-machine (M2M) interfaces In-depth examinations of internet of things (IoT) solutions in industry, including cloud computing IoT Perfect for professionals working in electrical industry sectors in manufacturing, production line manufacturers, engineers, and members of R&D industry teams, Electronics in Advanced Research Industries: Industry 4.0 to Industry 5.0 Advances will also earn a place in libraries of technicians working in the process industry. Öåíà: 15193.26 ðóá. ISBN: 9781119716891
Êóïèòü |
 Ðåêëàìà Litres.ru | Emerging Nanoelectronic Devices Àâòîð: Ãðóïïà àâòîðîâ Emerging Nanoelectronic Devices focuses on the future direction of semiconductor and emerging nanoscale device technology. As the dimensional scaling of CMOS approaches its limits, alternate information processing devices and microarchitectures are being explored to sustain increasing functionality at decreasing cost into the indefinite future. This is driving new paradigms of information processing enabled by innovative new devices, circuits, and architectures, necessary to support an increasingly interconnected world through a rapidly evolving internet. This original title provides a fresh perspective on emerging research devices in 26 up to date chapters written by the leading researchers in their respective areas. It supplements and extends the work performed by the Emerging Research Devices working group of the International Technology Roadmap for Semiconductors (ITRS). Key features: • Serves as an authoritative tutorial on innovative devices and architectures that populate the dynamic world of “Beyond CMOS” technologies. • Provides a realistic assessment of the strengths, weaknesses and key unknowns associated with each technology. • Suggests guidelines for the directions of future development of each technology. • Emphasizes physical concepts over mathematical development. • Provides an essential resource for students, researchers and practicing engineers. Öåíà: 11671.64 ðóá. ISBN: 9781118958261
Êóïèòü |
 Ðåêëàìà Litres.ru | Empowered Educators in Australia Àâòîð: Ann McIntyre BEST PRACTICES FROM AUSTRALIA'S HIGH-PERFORMING SCHOOL SYSTEMS Empowered Educators in Australia is one volume in a series that explores how high- performing educational systems from around the world achieve strong results. The anchor book, Empowered Educators: How High-Performing Systems Shape Teaching Quality Around the World, is written by Linda Darling-Hammond and colleagues, with contributions from the authors of this volume. The authors of Empowered Educators in Australia take an in-depth look at the policies and practices surrounding teaching quality in two different states: New South Wales (NSW) and Victoria. NSW offers significant support for government schools in areas such as staffing and teacher professional development. Victoria operates a highly devolved school system. Each provides a contrasting view of how federal and state policies combine to shape learning outcomes for students in Australia. The interplay between state and federal policy characterizes an intriguing «centralizing decentralization.» Initiatives to create national curricular, teaching, and teacher education standards all sit in balanced tension with a movement towards greater devolution of authority to schools. Together the NSW and Victoria case studies provide insights into policies that can support high-quality teaching in a federal education system. Australia's current educational reforms place increasing emphasis on issues of teaching quality, reshaping teaching as a standards-based, evidence-informed profession, and one that seeks to foster collegiality and professional exchange. These reforms encompass many aspects of a system that supports teaching quality, and highlight: the way teachers are trained, how they are inducted into the teaching profession and supported with mentors, the professional learning they receive, how they are appraised on their work, and the career pathways for teachers. Öåíà: 3042.2 ðóá. ISBN: 9781119369677
Êóïèòü |
 Ðåêëàìà Litres.ru | Enterprise Interoperability: Smart Services and Business Impact of Enterprise Interoperability Àâòîð: Ãðóïïà àâòîðîâ The ability of future industry to create interactive, flexible and always-on connections between design, manufacturing and supply is an ongoing challenge, affecting competitiveness, efficiency and resourcing. The goal of enterprise interoperability (EI) research is therefore to address the effectiveness of solutions that will successfully prepare organizations for the advent and uptake of new technologies. This volume outlines results and practical concepts from recent and ongoing European research studies in EI, and examines the results of research and discussions cultivated at the I-ESA 2018 conference, “Smart services and business impact of enterprise interoperability”. The conference, designed to encourage collaboration between academic inquiry and real-world industry applications, addressed a number of advanced multidisciplinary topics including Industry 4.0, Big Data, the Internet of Things, Cloud computing, ontology, artificial intelligence, virtual reality and enterprise modelling for future “smart” manufacturing. Readers will find this book to be a source of invaluable knowledge for enterprise architects in a range of industries and organizations. Öåíà: 16702.52 ðóá. ISBN: 9781119564027
Êóïèòü |
 Ðåêëàìà Litres.ru | Environment and Society Àâòîð: Paul Robbins Substantially updated for the second edition, this engaging and innovative introduction to the environment and society uses key theoretical approaches to explore familiar objects. Features substantial revisions and updates for the second edition, including new chapters on E waste, mosquitoes and uranium, improved maps and graphics, new exercises, shorter theory chapters, and refocused sections on environmental solutions Discusses topics such as population and scarcity, commodities, environmental ethics, risks and hazards, and political economy and applies them to objects like bottled water, tuna, and trees Accessible for students, and accompanied by in-book and online resources including exercises and boxed discussions, an online test bank, notes, suggested reading, and website links for enhanced understanding Offers additional online support for instructors, including suggested teaching models, PowerPoint slides for each chapter with full-color graphics, and supplementary images and teaching material Öåíà: 4261.45 ðóá. ISBN: 9781118451540
Êóïèòü |
 Ðåêëàìà Litres.ru | Enzyme Inhibition in Drug Discovery and Development. The Good and the Bad Àâòîð: Lu Chuang The science and applied approaches of enzyme inhibition in drug discovery and development Offering a unique approach that includes both the pharmacologic and pharmaco-kinetic aspects of enzyme inhibition, Enzyme Inhibition in Drug Discovery and Development examines the scientific concepts and experimental approaches related to enzyme inhibition as applied in drug discovery and drug development. With chapters written by over fifty leading experts in their fields, Enzyme Inhibition in Drug Discovery and Development fosters a cross-fertilization of pharmacology, drug metabolism, pharmacokinetics, and toxicology by understanding the «good» inhibitions—desirable pharmacological effects—and «bad» inhibitions—drug–drug interactions and toxicity. The book discusses: The drug discovery process, including drug discovery strategy, medicinal chemistry, analytical chemistry, drug metabolism, pharmacokinetics, and safety biomarker assessment The manipulations of drug metabolizing enzymes and transporters as well as the negative consequences, such as drug–drug interactions The inhibition of several major drug target pathways, such as the GPCR pathway, the NFkB pathway, and the ion channel pathway Through this focused, single-source reference on the fundamentals of drug discovery and development, researchers in drug metabolism and pharmacokinetics (DMPK) will learn and appreciate target biology in drug discovery; discovery biologists and medicinal chemists will also broaden their understanding of DMPK. Öåíà: 24738.54 ðóá. ISBN: 9780470538944
Êóïèòü |
 Ðåêëàìà Litres.ru | Enzymes and Drug Action Àâòîð: CIBA Foundation Symposium The Novartis Foundation Series is a popular collection of the proceedings from Novartis Foundation Symposia, in which groups of leading scientists from a range of topics across biology, chemistry and medicine assembled to present papers and discuss results. The Novartis Foundation, originally known as the Ciba Foundation, is well known to scientists and clinicians around the world. Öåíà: 11377.85 ðóá. ISBN: 9780470716762
Êóïèòü |
 Ðåêëàìà Litres.ru | Esophageal Cancer and Barrett's Esophagus Àâòîð: Ãðóïïà àâòîðîâ Esophageal Cancer and Barrett’s Esophagus, 3E, focuses on these two common and key conditions that affect the esophagus, providing expert guidance to their pathogenesis, cause, prevention, diagnosis and clinical management. Top international names in the field examine each of the many issues involved, using the very latest evidence-based research, and clear, didactic advice allows the reader to understand the best methods of diagnosis and clinical management of each condition – whether early or late stage. Well-illustrated and fully revised to include the latest in ACG/ASG/UEGW guidelines, it is the perfect consultation tool for gastroenterologists and oncologists managing patients with cancer of the esophagus. It is also ideal for teaching residents and fellows optimum patient management, and for identifying areas requiring future research. Öåíà: 10440.56 ðóá. ISBN: 9781118655191
Êóïèòü |
 Ðåêëàìà Litres.ru | Essential Manual of 24-Hour Blood Pressure Management Àâòîð: Kazuomi Kario Öåíà: 8025.73 ðóá. ISBN: 9781119799382
Êóïèòü |
 Ðåêëàìà Litres.ru | Essential Practical Prescribing Àâòîð: Victoria Taylor Essential Practical Prescribing is an important new textbook with a clinical, ward-based focus. It is specifically designed to help new foundation doctors working on the hospital wards and in the community, as well as medical students preparing for the Prescribing Safety Assessment. Using an accessible format, Essential Practical Prescribing demonstrates how to manage common medical conditions, and explains the logic behind each decision. It also emphasises common pitfalls leading to drug errors, and highlights drugs that could cause harm in certain situations. Organised by hospital department, it outlines the correct management of conditions, as well as highlighting the typical trials of a junior doctor. Essential Practical Prescribing: Contains a range of learning methods within each chapter including: key topics, learning objectives, case studies, DRUGS checklists, «Top-Tips», advice on guidelines and evidence, and key learning points Uses patient histories to set the scene and enhance the clinical emphasis Offers examples of correctly completed drug charts throughout, which are also available online Is an ideal companion for Prescribing Safety Assessment (PSA) preparation Includes a companion website at www.wileyessential.com/prescribing featuring MCQs and downloadable DRUGS checklists and drug charts Öåíà: 5551.72 ðóá. ISBN: 9781118837702
Êóïèòü |
 Ðåêëàìà Litres.ru | Essentials of Inorganic Chemistry. For Students of Pharmacy, Pharmaceutical Sciences and Medicinal Chemistry Àâòîð: Katja Strohfeldt A A comprehensive introduction to inorganic chemistry and, specifically, the science of metal-based drugs, Essentials of Inorganic Chemistry describes the basics of inorganic chemistry, including organometallic chemistry and radiochemistry, from a pharmaceutical perspective. Written for students of pharmacy and pharmacology, pharmaceutical sciences, medicinal chemistry and other health-care related subjects, this accessible text introduces chemical principles with relevant pharmaceutical examples rather than as stand-alone concepts, allowing students to see the relevance of this subject for their future professions. It includes exercises and case studies. Öåíà: 7732.52 ðóá. ISBN: 9781118695388
Êóïèòü |
 Ðåêëàìà Litres.ru | Evaluating Pharmaceuticals for Health Policy and Reimbursement Àâòîð: Nick Freemantle “The challenge in all settings is to make the difficult decisions in a way that is defensible, justifiable, ethical, and equitable” So write Nick Freemantle and Suzanne Hill in their introduction to this important discussion on decision making in the reimbursement of pharmaceuticals. Based around a programme supported by the World Health Organization, chapters by leading academics involved in the research tackle such major issues as international pharmaceutical policy, tensions in licensing policies, priority setting, and relationships between the stakeholders. Chapters include Development of marketing authorisation procedures for pharmaceuticals Interpreting clinical evidence International pharmaceutical policy: health creation or wealth creation? Development of fourth hurdle policies around the world Economic modelling in drug reimbursement Priority setting in health care: matching decision criteria with policy objectives Tensions in licensing and reimbursement decisions: case of riluzole for amytrophic lateral sclerosis Relationship between stakeholders: managing the war of words Medicine and the media: good information or misleading hype? How to promote quality use of cost-effective medicines Using economic evaluation to inform health policy and reimbursement: making it happen and making it sustainable Pricing of pharmaceuticals Evaluating pharmaceuticals for health policy in low and middle income country settings. Besides the controversial issues there is a wealth of practical information including economic modelling and the experiences from the WHO programme, providing readers with workable examples. This is essential reading for clinical researchers in pharmaceuticals and policy makers everywhere. Öåíà: 14686.26 ðóá. ISBN: 9781405140973
Êóïèòü |
 Ðåêëàìà Litres.ru | Evaluation of Drug Candidates for Preclinical Development Àâòîð: Ãðóïïà àâòîðîâ Emphasizes the integration of major areas of drug discovery and their importance in candidate evaluation It is believed that selecting the «right» drug candidate for development is the key to success. In the last decade, pharmaceutical R&D departments have integrated pharmacokinetics and drug metabolism, pharmaceutics, and toxicology into early drug discovery to improve the assessment of potential drug compounds. Now, Evaluation of Drug Candidates for Preclinical Development provides a complete view and understanding of why absorption-distribution-metabolism-excretion-toxicology (ADMET) plays a pivotal role in drug discovery and development. Encompassing the three major interrelated areas in which optimization and evaluation of drug developability is most critical—pharmacokinetics and drug metabolism, pharmaceutics, and safety assessment—this unique resource encourages integrated thinking in drug discovery. The contributors to this volume: Cover drug transporters, cytochrome P-450 and drug-drug interactions, plasma protein binding, stability, drug formulation, preclinical safety assessment, toxicology, and toxicokinetics Address developability issues that challenge pharma companies, moving beyond isolated experimental results Reveal connections between the key scientific areas that are critical for successful drug discovery and development Inspire forward-thinking strategies and decision-making processes in preclinical evaluation to maximize the potential of drug candidates to progress through development efficiently and meet the increasing demands of the marketplace Evaluation of Drug Candidates for Preclinical Development serves as an introductory reference for those new to the pharmaceutical industry and drug discovery in particular. It is especially well suited for scientists and management teams in small- to mid-sized pharmaceutical companies, as well as academic researchers and graduate students concerned with the practical aspects related to the evaluation of drug developability. Öåíà: 13459.08 ðóá. ISBN: 9780470574881
Êóïèòü |